Funkční antibakteriální ponožky nanosox® AGTIVE COMFORT INVISIBLE pro vás všechny, kteří kladete vysoké nároky na zdraví svých nohou a zdravé prostředí v obuvi. Nanosox Comfort jsou určeny do každého typu obuvi, pro běžné nošení v práci, kanceláři i ve volném čase. Velmi dobře splní svou funkci ve sportovní obuvi v chladném, ale také v teplém období díky unikátní směsi vláken.
Použitá směs jemných vlněných vláken Merino a technických vláken velmi dobře transportuje pot od pokožky, nevzniká mokré prostředí v botě, nestudí na pokožce a zajišťuje tepelný komfort vašim nohám. Konstrukce ponožky je typická svými aktivními zónami - různými úplety, charakterizující jednotlivé zóny:
- zesílená pata a špička
- kompresní pás přes nárt
- pevný / nesvíravý lem
- číslovaní na vnitřní straně lemu
Účinné látky, tedy ionty stříbra, jsou nesepratelné, protože jsou obsaženy v celém objemu nosného vlákna, jsou bezpečné, nedráždí vaši pokožku a nezpůsobují alergie.
Stříbro aktivně omezuje množení bakterií a tím poskytuje trvalou a dlouhodobou ochranu proti zápachu. NanoSox Comfort nemusíte po každém použití prát a tím aktivně přispíváte k ochraně životního prostředí :)
MADE IN CZ - Ručně vyrobeno v České republice
Použitý materiál:
35% merino vlna, 9% polyamid s ionty stříbra (PAD), 36% polypropylen (PP), 20% elastan
Technické detaily:
- příjemný pocit a komfort, teplé a prokrvené nohy
- eliminaci tvorby nepříjemného zápachu
- úlevu nohám se sklonem k mykózám a plísňovým onemocněním
- aktivní ničení bakterií a schopnost rychlého odvodu vlhkosti a potu
- prodyšnost, hebkost a pružnost
- nedráždivost a vysokou snášenlivost
Nanosox Comfort Plus jsou revoluční protože:
- obsahují nanočástice- ionty stříbra
- kombinují vlnu, polypropylén, elastan a polyamid = větší pevnost
- poskytují trvalou atibakteriální ochranu – stále účinné
- zesílenou patu a špičku, kompresní pásek přes nárt
- vysoký lem jemně sevře a zamezí posunu v botě
- označení velikosti ulehčí párování po vyprání
Použití:
Vhodné do: uzavřené trekové, sportovní nebo lyžařské obuvi, pro motosport, treking, cestování a všechny outdoorové aktivity a pobyt v přírodě v chladném počasí
Anatomicky tvarované antibakteriální ponožky pro Vás všechny, kteří jste si oblíbili sport a aktivity v chladném počasí nebo cestování, kdy se teploty střídají.
Údržba:
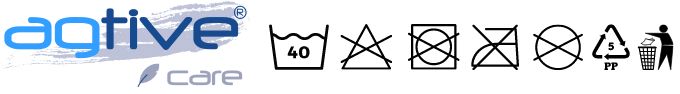
- Dbejte důsledně pokynů na etiketách jednotlivých výrobků, ošetřujte je dle zobrazených symbolů.
- Používejte prací lázeň o teplotě max. 40°C, vyšší teplota může oděvy poškodit. Naše materiály jsou antibakteriální a odolné ušpinění, vyšší teplota není nutná. Pokud jsou oděvy znečištěné od prachu, neodkládejte jejich vyprání, prach poškozuje strukturu vláken.
- Doporučujeme prát oděvy obrácené na rubovou stranu, jejich estetika bude zachována po delší dobu. Perte odděleně od ostatního prádla, jejich strukturu mohou poškodit zipy a kovové součásti na ostatních oděvech.
- Používejte běžné prací prostředky, nejlépe se osvědčily tekuté prací přípravky bez aviváže. Nenechávejte prádlo dlouho namočené v pracím prostředku. Nepoužívejte aviváž. Aviváž vytváří film na jednotlivých vláknech, brání antibakteriální funkci a ucpává kapilární systém odvodu potu.
- Oděvy sušte volně zavěšené; rychle vysychají díky své nenasákavosti; nepoužívejte sušičku ani jiná topná tělesa.
- Materiály, používané k výrobě našich oděvů, se nemačkají, proto žehlení není nutné.
Číslování a velikosti - ponožky
| Dětské | Dámské | Pánské | ČSN (CZ) vnitřní délka [mm] | ČSN (CZ) vnější délka [cm] | UK anglické [inch] | EU francouzské [steh] |
| ✔︎ | | | 205 | 21,5 | 1 | 33 |
| ✔︎ | ✔︎ | | 215 | 22,5 | 2 | 34 |
| ✔︎ | ✔︎ | | 220 | 23 | 3 | 35 |
| ✔︎ | ✔︎ | | 225 | 23,5 | 3,5 | 36 |
| ✔︎ | ✔︎ | | 230 | 24 | 4 | 37 |
| | ✔︎ | ✔︎ | 240 | 25 | 5 | 38 |
| | ✔︎ | ✔︎ | 250 | 26 | 6 | 39 |
| | ✔︎ | ✔︎ | 255 | 26,5 | 6,5 | 40 |
| | ✔︎ | ✔︎ | 260 | 27 | 7 | 41 |
| | ✔︎ | ✔︎ | 270 | 28 | 8 | 42 |
| | | ✔︎ | 280 | 29 | 9 | 43 |
| | | ✔︎ | 285 | 29,5 | 9,5 | 44 |
| | | ✔︎ | 290 | 30 | 10 | 45 |
| | | ✔︎ | 295 | 30,5 | 11 | 46 |
| | | ✔︎ | 300 | 31 | 12 | 47 |
| | | ✔︎ | 310 | 32 | 13 | 48 |